In the rapidly evolving landscape of pharmaceutical manufacturing, the integration of cutting-edge technologies is not just a trend but a necessity to meet the stringent regulatory standards and ensure the highest quality of products. The advent of Pharma 4.0 has ushered in a new era of digital transformation, leveraging the power of the Internet of Things (IoT) to revolutionize traditional processes. Among the myriad applications of IoT in the pharmaceutical industry, its impact on cleanroom monitoring stands out as a game-changer, significantly enhancing operational efficiency, compliance, and product quality. This blog delves into how IoT is elevating cleanroom performance, marking a pivotal shift towards smarter, more reliable, and efficient pharmaceutical manufacturing.
Understanding the Role of Cleanrooms in Pharmaceutical Manufacturing
Cleanrooms are controlled environments with low levels of pollutants such as dust, airborne microbes, aerosol particles, and chemical vapors. They are critical in pharmaceutical manufacturing, where the sterility and quality of products must be maintained to meet regulatory requirements and ensure patient safety. Traditional cleanroom monitoring methods involve manual sampling and testing, which can be time-consuming, labor-intensive, and prone to human error. Enter the IoT, which promises to transform this scenario by automating and enhancing monitoring processes.
The IoT Revolution in Cleanroom Monitoring
IoT technology involves a network of sensors, devices, and systems connected to the internet, capable of collecting, transmitting, and analyzing data in real-time. When applied to cleanroom environments, IoT offers unparalleled advantages, ushering in a new level of precision, efficiency, and control. Here’s how IoT is making a difference:
1. Real-time Monitoring and Control
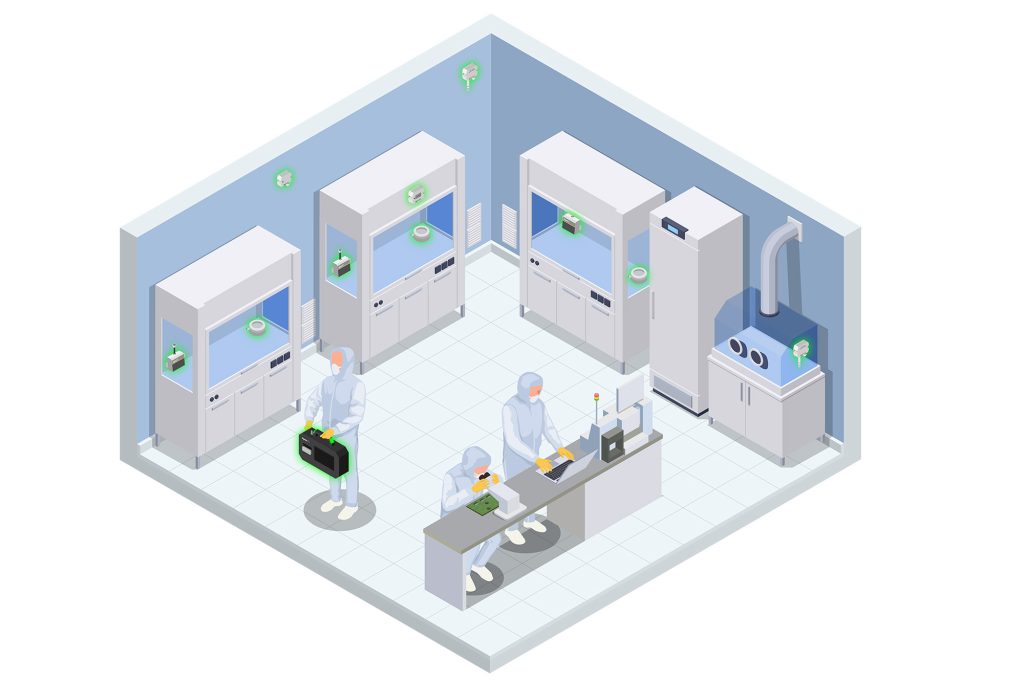
IoT-enabled sensors can continuously monitor various environmental parameters such as temperature, humidity, particulate matter, and microbial contamination levels. This real-time data collection allows for immediate adjustments to maintain optimal conditions, significantly reducing the risk of contamination and ensuring compliance with regulatory standards.
2. Predictive Maintenance
IoT devices can predict equipment failures before they occur by analyzing data trends and identifying anomalies. This predictive maintenance capability can prevent downtime, reduce repair costs, and extend the lifespan of critical cleanroom equipment such as HVAC systems, air filters, and sterilization units.
3. Enhanced Data Integrity and Traceability
With IoT, every piece of data collected is automatically logged and stored in secure cloud-based systems. This automation not only minimizes the risk of data tampering or loss but also ensures complete traceability of all monitoring activities. Such robust data integrity is crucial for meeting Good Manufacturing Practice (GMP) requirements and facilitating audits.
4. Energy Efficiency and Sustainability
IoT technology can optimize the use of resources in cleanrooms, such as electricity and water, by monitoring consumption patterns and adjusting operations accordingly. This not only reduces operational costs but also supports the pharmaceutical industry’s growing commitment to sustainability.
5. Integration with Pharma 4.0 Systems
IoT is a cornerstone of the Pharma 4.0 initiative, which aims to integrate digital technologies into all aspects of pharmaceutical manufacturing. By connecting cleanroom monitoring systems with other Pharma 4.0 components, such as advanced analytics, artificial intelligence (AI), and digital twins, manufacturers can gain deeper insights, improve decision-making, and innovate faster.
Case Studies: IoT in Action
Several leading pharmaceutical companies have already begun reaping the benefits of IoT in their cleanroom operations. For instance, a global pharma giant implemented IoT sensors for continuous environmental monitoring, resulting in a 20% reduction in contamination incidents and a 15% decrease in energy consumption within the first year of deployment. Another example is a biotech firm that used IoT-based predictive maintenance to identify and fix a critical air handling unit issue, avoiding a potential shutdown that could have delayed product delivery by weeks.
Overcoming Challenges
While the benefits of IoT in cleanroom monitoring are clear, its implementation is not without challenges. Concerns about cybersecurity, data privacy, and the initial investment in technology can be significant hurdles. However, with robust security protocols, clear regulatory compliance, and a strategic approach to digital transformation, these challenges can be effectively managed.
The Future of Cleanroom Monitoring
As the pharmaceutical industry continues to embrace digital transformation, the role of IoT in cleanroom monitoring is set to grow exponentially. Future advancements may include more sophisticated sensors, AI-driven predictive analytics, and even greater integration with other digital technologies, further enhancing the efficiency, reliability, and compliance of cleanroom operations.
Conclusion
The integration of IoT technology into cleanroom monitoring represents a significant leap forward in the quest for excellence in pharmaceutical manufacturing. By enabling real-time monitoring, predictive maintenance, enhanced data integrity, and energy efficiency, IoT elevates cleanroom performance to new heights. As we move deeper into the era of Pharma 4.0, the potential of IoT to transform traditional processes and drive innovation is boundless. Embracing this digital revolution is not just an option but a necessity for pharmaceutical manufacturers aiming to stay competitive and meet the evolving demands of regulatory authorities and patients alike. The journey towards smarter, more efficient, and compliant cleanroom operations is well underway, with IoT leading the charge.